Insights+: EMA Marketing Authorization of New Drugs in July 2023
Shots:
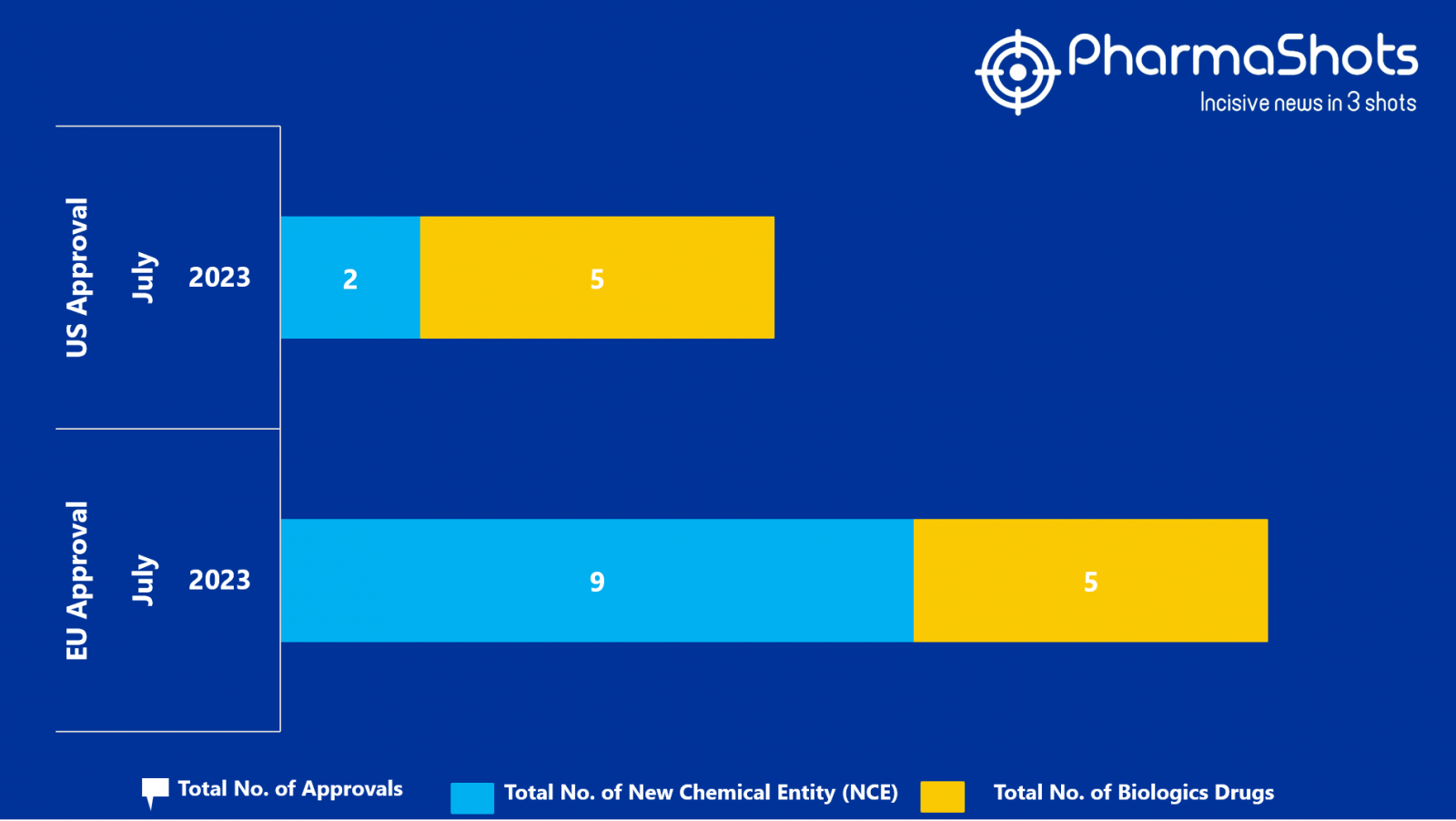
- The EMA approved 9 New Chemical Entity (NCE) and 5 Biologic Drugs in July 2023, leading to treatments for patients and advances in the healthcare industry
- In July 2023, the major highlights drugs were Soliris approval for refractory generalised myasthenia gravis and Trodelvy for pre-treated HR+/HER2- metastatic breast cancer
- PharmaShots has compiled a list of a total of 14 new drugs approved by the EMA in July 2023
Lytgobi
Active ingredient: futibatinib Approved: July 06, 2023
Company: Taiho Oncology Europe GmbH Disease: Cholangiocarcinoma
- The EC has granted conditional marketing authorization for Lytgobi monotx. to treat adult patients with LA or metastatic CCA with an FGFR2 fusion or rearrangement that have progressed after one prior line of systemic therapy
- The conditional marketing authorization was based on the (FOENIX-CCA2) open-label trial evaluating Lytgobi (20mg, qd) in 103 patients with unresectable, LA or metastatic intrahepatic CCA harboring FGFR2 gene rearrangements, incl. fusions
- The results showed that patients treated with Lytgobi achieved an ORR of 42% as assessed by independent review and m-DoR (9.7mos.) while 72% of the responses lasted >6mos.
2. Novavax’s Nuvaxovid Receives EC’s Marketing Authorization for the Prevention of COVID-19
Novavax
Active ingredient: NVX-CoV2373 Approved: July 07, 2023
Company: Novavax Disease: COVID-19
- The EC has granted full marketing authorization for Nuvaxovid (NVX-CoV2373), following a positive opinion from the EMA’s CHMP. The vaccine can now be used as a primary series in individuals aged ≥12yrs. and as a booster dose in adults aged ≥18yrs. to prevent COVID-19,
- The P-III trial (PREVENT-19) results demonstrated the safety profile as well as efficacy as a primary series in adults, the immunogenicity and safety as a booster dose in adults, and the efficacy and safety as a primary series in individuals aged ≥12yrs.
- Novavax’s Nuvaxovid is authorized for use in 40+ markets globally. The company also plans to file for full approval in the US as well as other markets
Columvi
Active ingredient: glofitamab Approved: July 11, 2023
Company: Roche Disease: Diffuse Large B-cell Lymphoma
- Columvi has received conditional marketing authorization from the EC for adults with r/r DLBCL. The approval was based on the P-I/II (NP30179) study evaluating Columvi in 860 patients
- The results showed that Columvi gave as a fixed course induced a complete response (35.2%), ORR (50%), 74.6% continued to experience a response at 12mos., the median duration of CR (not reached), median follow-up for DoR (12.8mos.), median time to first CR was 42 days, CRS was low grade (grade 1: 48.1%; grade 2: 12.3%), CRS leading to treatment discontinuation in 1 patient
- Additional study results from a larger cohort reinforce the durability of Columvi. The company continues to expand the clinical development program incl. the P-III trial (STARGLO) of Columvi + GemOx for 2L+ DLBCL
Talvey and Tecvayli
Active ingredient: talquetamab and teclistamab Approved: July 21, 2023
Company: Janssen Disease: Multiple Myeloma
- The EMA’s CHMP has adopted the positive opinion recommended conditional marketing authorization for Talvey as monotx. for adult patients with RRMM who have received 3 prior therapies and also recommended the approval of a Type II variation for teclistamab
- The recommendation was based on the results from the P-I/II study (MonumenTAL-1) presented at ASCO and EHA 2023 and P-I/II trial (MajesTEC-1) presented at ASCO 2023 evaluating talquetamab and teclistamab
- Talquetamab, a bispecific T-cell engaging Ab, and Teclistamab, an off-the-shelf bispecific Ab which are currently being studied in multiple monotx. & combination studies. Talquetamab received BTD from the US FDA in June 2022 & ODD from the EMA in Aug 2021 and from the US FDA in May 2021
5. Merck Receives EMA’s CHMP Positive Opinion Recommending Approval of Gefapixant for Chronic Cough
Lyfnua
Active ingredient: Gefapixant Approved: July 21, 2023
Company: Merck Disease: Chronic Cough
- The EMA’s CHMP has recommended the approval of gefapixant, a non-narcotic, oral selective P2X3 receptor antagonist for adults with refractory or unexplained chronic cough. The EC will review the CHMP’s recommendation for marketing authorization in the EU & a final decision is expected in 2023
- The opinion was based on the P-III (COUGH-1 & 2) trial results evaluating gefapixant vs PBO in 2044 patients. Both studies showed that adults treated with gefapixant (45mg, BID) achieved an 18.45% reduction in 24hr. cough frequency at 12wks. in (COUGH-1) while 14.64% in (COUGH-2) trial at 24wks.
- Gefapixant (15mg, BID) did not meet the primary efficacy EPs & results were published in The Lancet. Gefapixant was approved in Japan & Switzerland under the brand name Lyfnua for refractory or unexplained chronic cough
Tevimbra
Active ingredient: tislelizumab Approved: July 21, 2023
Company: BeiGene Disease: Esophageal Squamous Cell Carcinoma
- The EMA’s CHMP has issued a positive opinion recommending approval of tislelizumab as monotx. for adult patients with unresectable, LA or metastatic ESCC after prior Pt-based CT
- The MAA was based on the P-III study (RATIONALE 302) evaluating tislelizumab (humanized IgG4 anti-PD-1 mAb) vs CT in 513 patients from 132 research sites in 11 countries in Asia, EU & North America. The study met its 1EPs & showed benefit, m-OS was 8.6 vs 6.3mos. & the safety profile was consistent with prior trials
- The MAA also incl. safety data for 1972 patients who received tislelizumab monotx. in 7 clinical trials. Additionally, regulatory submissions for tislelizumab are under review by authorities in the US, UK, Australia, China, New Zealand, Brazil, Korea, Switzerland & Indonesia
Tepkinly
Active ingredient: epcoritamab Approved: July 21, 2023
Company: AbbVie Disease: Diffuse Large B-cell Lymphoma
- The EMA’s CHMP has adopted a positive opinion recommending conditional marketing authorization for epcoritamab as monotx. for adult patients with r/r DLBCL after two or more lines of systemic therapy. The EC’s decision is expected in 2023
- The opinion was based on the P-I/II open-label trial (EPCORE NHL-1) evaluating epcoritamab in patients with relapsed, progressive or refractory CD20+ mature B-cell NHL incl. DLBCL. The therapy is being co-developed by AbbVie & Genmab
- The 1EPs of the study was ORR (63.1%) as assessed by IRC. If epcoritamab is approved, it will become 1st SC bispecific Ab conditionally approved as a monotx. for r/r DLBCL & will be marketed under the brand name Tepkinly in all EU member states, Liechtenstein, Norway, and Iceland
8. ViiV Healthcare Receives EMA’s CHMP Positive Opinion of Cabotegravir for HIV Prevention
Vocabria
Active ingredient: cabotegravir Approved: July 24, 2023
Company: ViiV Healthcare Disease: HIV
- The EMA’s CHMP has adopted the positive opinion recommending marketing authorization for cabotegravir long-acting (LA) injectable and tablets for HIV prevention
- The positive opinion was based on the 2 international P-IIb/III studies (HPTN 083 & 084) evaluating cabotegravir (q8w) for PrEP in HIV- men who have sex with men, transgender women & cisgender women who were at increased risk of acquiring HIV
- The studies showed that cabotegravir LA for PrEP was superior to emtricitabine/tenofovir disoproxil fumarate (FTC/TDF), with 69% & 90% lower rates of HIV acquisition over FTC/TDF tablets in (HPTN 083) & (HPTN 084) studies. Cabotegravir for PrEP was approved in the US, Australia, Zimbabwe, South Africa, Malawi, Botswana & Brazil as Apretude
Jardiance
Active ingredient: empagliflozin Approved: July 26, 2023
Company: Boehringer Ingelheim and Eli Lilly Disease: Chronic Kidney Disease
- The EC has approved Jardiance for adults with CKD. The approval was based on the P-III trial (EMPA-KIDNEY) evaluating the effect of Jardiance in 6609 adults from 8 countries
- The results showed a significant benefit of empagliflozin in reducing the relative risk of kidney disease progression or cardiovascular death by 28% with an absolute risk reduction of 3.8%, 14% relative risk reduction in hospitalization for any cause with absolute risk reduction (4.4%). The overall safety data was consistent with prior results confirming the well-established safety profile of empagliflozin
- Jardiance, the first SGLT2 inhibitor to show a significant reduction in all-cause hospitalizations. The product is expected to be available as quickly as possible
Camzyos
Active ingredient: mavacamten Approved: July 27, 2023
Company: BMS Disease: Symptomatic Obstructive Hypertrophic Cardiomyopathy
- The EMA’s CHMP has recommended approval of Camzyos (cardiac myosin inhibitor) for symptomatic (New York Heart Association, NYHA, class II-III) obstructive HCM
- The opinion was based on 2 P-III trials (EXPLORER-HCM) & (VALOR-HCM) evaluating Camzyos vs PBO in 251 & 112 adult patients. Both trials met their 1EPs & 2EPs & showed a clear treatment effect in the P-III trial (EXPLORER-HCM) with clinical improvements in exercise capacity, symptoms, and functional status along with an improvement in LVOTO
- The P-III trial (VALOR-HCM) showed an improvement across cardiac measures who met the 2011 ACC/AHA or 2014 ESC guideline criteria for SRT & were referred for an invasive procedure. The therapy also showed a reduction in need or eligibility for SRT
Soliris
Active ingredient: eculizumab Approved: July 27, 2023
Company: AstraZeneca Disease: Generalised Myasthenia Gravis
- The EC has approved Soliris (C5 complement inhibitor) for expanded use to include the treatment of refractory gMG in children & adolescents aged 6-17yrs. who are AChR Ab+. The approval was based on the P-III trial of Soliris in 11 patients aged 12-17yrs. with refractory gMG
- The results showed a clinical benefit in paediatric patients who previously failed immunosuppressive treatment & experienced significant unresolved disease symptoms, improvement in 1EPs of change from baseline in QMG total score were seen at 26wk.
- The efficacy & safety of Soliris was consistent with the established profile of Soliris in clinical trials incl. adults with refractory gMG. Soliris was approved in the US, EU & Japan for adults with NMOSD
Trodelvy
Active ingredient: sacituzumab govitecan Approved: July 28, 2023
Company: Gilead Disease: Breast Cancer
- The EC has approved Trodelvy as a monotx. for adult patients with unresectable or metastatic HR+ HER2- breast cancer who have received endocrine-based therapy & 2 additional systemic therapies in the advanced setting
- The approval was based on the P-III study (TROPiCS-02) evaluating Trodelvy vs CT in a ratio (1:1) in 543 patients, showed a significant & clinical OS benefit (3.2mos.), m-OS (14.4 vs 11.2mos.), 34% reduction in risk of disease progression or death, m-PFS (5.5 vs 4.0mos.) people were progression-free at 1yr. (21% vs 7%)
- Improvement in additional 2EPs measures, incl. ORR, TTD with no significant difference in TTD in Pain Scale. The safety profile was consistent with prior studies with no new safety signals, adverse reactions leading to treatment discontinuation (6% vs 4%)
13. Curium’s Pylclari Receives EC’s Marketing Authorization for Adults with Prostate Cancer
Pylclari
Active ingredient: piflufolastat (18F) Approved: July 28, 2023
Company: Curium Disease: Prostate Cancer
- The EC has granted marketing authorization to Pylclari, an innovative 18F-PSMA PET tracer for the detection of PSMA+ lesions with PET in adults with prostate cancer
- The PSMA-PET tracer is indicated for the following clinical settings: Primary staging of patients with high-risk prostate cancer prior to initial curative therapy & to localize recurrence of prostate cancer in patients with a suspected recurrence based on increasing serum PSA levels after primary treatment with curative intent
- In May 2021, the US FDA approved piflufolastat F 18 (Pylarify) inj. to identify suspected metastasis or recurrence in patients with prostate cancer, based on data from the P-III trials (OSPREY) & (CONDOR)
Ztalmy
Active ingredient: ganaxolone Approved: July 31, 2023
Company: Marinus Disease: Epileptic Seizures
- The EC has approved Ztalmy oral suspension for adjunctive treatment of epileptic seizures associated with CDKL5 deficiency disorder (CDD) in patients aged 2-17yrs.
- The approval was based on the P-III trial (Marigold) evaluating Ztalmy, a neuroactive steroid GABAA receptor modulator in 101 patients. The trial met the 1EPs & showed a 30.7% median reduction in 28-day major motor seizure frequency over 6.9% in PBO
- In the (Marigold) OLE study, patients experienced a median 49.6% reduction in major motor seizure frequency for 12mos. & showed efficacy, safety & tolerability with the most common adverse reactions & the results were published in The Lancet Neurology
Related Post: Insights+: EMA Marketing Authorization of New Drugs in June 2023




